Project Description
Understanding the Circular Economy and What Hospitals Can Do to Grow It
We learn from the earliest age that nature is circular. When a plant or animal is born it enters a circle of life.
But in the industrialized world, when a new cell phone model is released or a refrigerator is broken “beyond repair,” we are conditioned to buy anew. Each new product requires raw materials to be extracted from the earth. Additional non-renewable resources are used to manufacture, assemble, then ship the product. This “linear” model is sometimes called “take-make-waste,” because we take from the Earth, make and use the product, then throw it in the trash.
But what if the goods of today become the resources of tomorrow, where products used once could be disassembled, fixed, and reassembled? As in nature, we would use products in a more sustainable, circular way.
In a circular economy, products are created using business models designed to maintain them at the highest level of functionality for as long as possible, through reprocessing or remanufacturing, among other strategies. Using products repeatedly typically results in lower greenhouse gas emissions than using a new or original product each time. Plus, reducing the demands on new products lessens dependence on a global supply chain, cuts waste, and reduces costs.
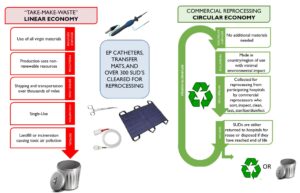
Using reprocessed (sometimes known as “remanufactured” in Europe) “single-use” medical devices drives a circular economy solution. A well-designed, peer-reviewed, published study found that use of reprocessed devices reduces environmental impact in 13 of 16 categories, including cutting in half (or more) climate changing and ozone depleting emissions compared with using an original device (Sustainability, 2021).
This is especially important for the health sector, where greenhouse gas emissions originate overwhelmingly from the supply chain (Health Affairs, 2020). When hospitals use commercially reprocessed devices, they drive the circular economy.
Products labelled for “single-use” are linear. When it comes to healthcare, the commercial reprocessing of “single-use” medical devices (SUDs) is the only pathway the U.S. Food and Drug Administration (FDA), and related oversight authorities, regulates that allows hospitals to safely and efficiently convert linear products to circular.
The UK’s National Health Service (NHS) became the world’s first national health system to commit to becoming “carbon net zero” – a goal that identified its supply chain as a high value target for emission reduction. Since 2020, NHS has removed 200,000 single-use plastic items from its waste stream, saving four tons of waste per year (Modern Healthcare, March 2021). NHS has identified SUD remanufacturing programs as critical to achieving net zero.
In addition to reducing waste and emissions, improving supply chain resilience, using commercially reprocessed medical devices results in substantial cost savings, as reprocessed devices typically cost 25 to 40% less than the original devices. Savings can be used to fund additional environmental mitigation efforts.
Turning the Light Green: Resources for Increasing Sustainable Practices
Hospitals worldwide are embracing medical device reprocessing programs as an immediate, regulated means to quantifiably reduce emissions, waste, and cost while improving supply chain dependability. AMDR urges swift action by hospitals and surgical centers to work with commercial reprocessors to maximize programs to incur these benefits.
Further, by seeking a commitment in writing from hospital leadership, sustainability managers provide a roadmap needed to affect change. Please see a template for creating a Reprocessing Sustainable Standard document that leadership can review and approve. Be sure to see AMDR’s Best Practices from Top Reprocessing Hospitals for additional recommendations.
AMDR is also working to ensure SUD reprocessing and remanufacturing are included in forthcoming Sustainable purchasing guides, such as Practice Greenhealth’s Sustainable Procurement Guide, which include a calculator for reducing greenhouse gas emissions in the supply chain. As new data become available on emissions caused or saved by use of particular devices, the guide is updated. Turn to these guides to learn about the full range of tools available to reduce your hospital’s carbon footprint.
Over 300 types of SUDs have clearance from the FDA, and are CE marked in the EU. Commonly reprocessed SUDs include non-invasive devices such as transfer mats, sequential compression sleeves, tourniquet cuffs, and pulse oximeter sensors as well as minimally invasive surgical devices including laparoscopic graspers, scissors, forceps, scalpels, orthopedic blades, bits, burs, external fixation clamps, and electrophysiological cardiac catheters.
AMDR members are pleased to discuss SUDs that could be reprocessed from hospitals, surgical centers, and doctors’ offices. Members include Arjo ReNu Medical, Innovative Health, Medline ReNewal, Stryker’s Sustainability Solutions, Sustainable Technologies (a Cardinal Health Business), Vanguard AG, and Vein 360.
Look for new app-based tools entering the marketplace that can help calculate the reduction in greenhouse gas emissions by using reprocessed devices as much as possible. We will update this space with resources as they become available.
{Your hospital or surgical center} is committed to sourcing sustainable goods and services. This standard identifies our commitment to reprocess and buy back SUDs. This standard defines the scope of goods covered and the standard we will follow whenever possible.
In Scope:
This standard applies to SUDs cleared by the FDA (or appropriate regulatory authority in other countries) for commercial reprocessing.
Out of Scope:
SUDs that regulatory authorities have not cleared for reprocessing; reprocessed SUDs that may not be purchased due to contract commitments or other contract compliance issues.
Definitions:
Reprocessing: Reprocessing includes the steps performed by commercial reprocessors to make a contaminated reusable or SUD ready for patient use. The steps may include decontamination, cleaning, functional testing, repackaging, relabeling, disinfection, or sterilization.
“Single-use” devices (SUDs): Also referred to as disposable devices, these are intended for use on one patient during a single procedure. Note that there is no regulatory requirement to designate a device for single use. Instead, each original equipment manufacturer (OEM) makes the designation. In many cases this label is appropriate. However, over 300 types of SUDs are cleared by the FDA and/or CE marked in the EU.
Commercial reprocessor: A business that reprocesses used SUDs for another party is required to assume full product liability and meet the medical device manufacturer requirements of the FDA or other federal regulatory authorities.
Sustainability standard:
- {Your organization} will maximize opportunities to reprocess SUDs and buy back reprocessed devices to support reuse and resource conservation.
- {Your organization} seeks services that offer opportunities to reprocess non-critical or semi-critical SUDs where devices are disinfected or sterilized with alternatives to ethylene oxide (ETO) while continuing to meet health and safety standards.
- {Your organization} will prioritize reprocessing over recycling, given its priority in waste reduction. Nevertheless, {Your organization} will seek services aimed at reducing waste in a sustainable manner (such as by recycling, OEM Take-Backs, etc., to avoid landfill and incineration as much as possible) from SUDs that have reached their end of life or are not able to be reprocessed.
- {Your organization} seeks services for measuring, reporting, and reducing their greenhouse gas emissions including reports from suppliers on the total carbon footprint of their products and/or Life Cycle Assessments as compared to reusable or reprocessable alternatives to disposable devices.
- {Your organization} seeks services that support diversity and inclusion.

