What difference can one person make? When it comes to reducing greenhouse gas emissions from hospitals, anyone can make a huge difference!
AMDR has created this toolbox as a resource page for those interested in advancing policies that encourage the use of more reprocessed devices – whether that change is at your hospital, or at the federal policy level. All of these materials are free for you to download and use as you wish.
Need something that’s not here? Drop us a line at info@amdr.org. We would love to hear from you and learn more ways we can support you.
Sample Reprocessing Slide Deck
If interested in receiving slide deck, please email DSheon@amdr.org.
Key Messages
- Reprocessed single-use medical devices reduces greenhouse gas emissions, cost, waste, and strengthens the supply chain.
- HHS/AHRQ and the National Academy of Medicine are urging hospitals to increase the use of reprocessed single-use devices as a key strategy to reduce hospital greenhouse gas emissions.
- Although nearly 9,400 hospitals reprocess at least some single-use devices, experts believe that only a small portion of the devices that FDA regulates for reprocessing are being safely reused. If we want to reduce greenhouse gas emissions, costs, and waste, we need to do better.
- The FDA regulates reprocessing for over 300 types of devices labelled for single-use. These devices are as safe and effective as the original equipment.
- Those looking for more information can go to ResponsibleReuse.org.
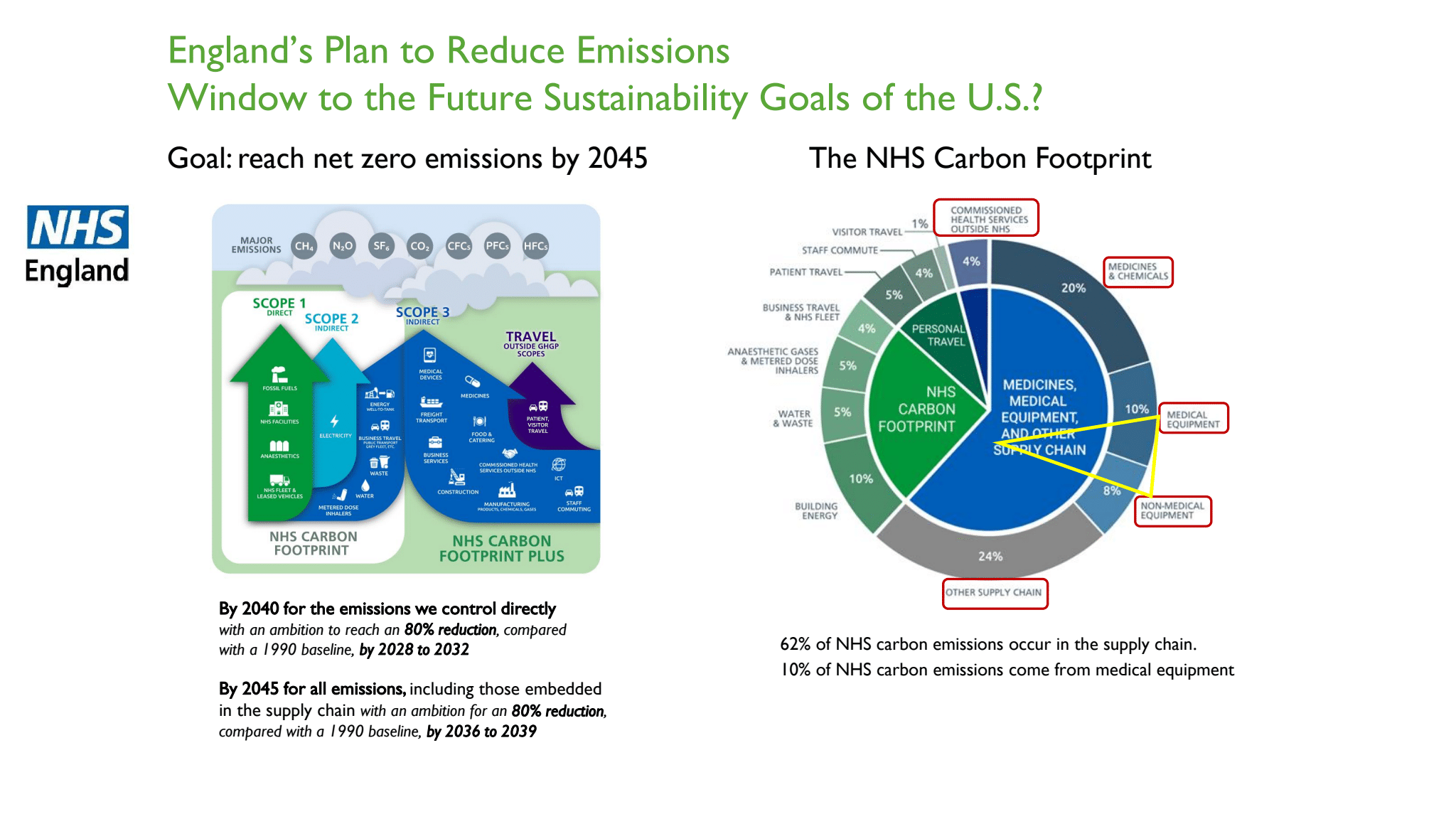
- Unprecedented research and Life Cycle Assessments published since COVID show significantly lower emissions associated with R-SUDs. Emissions from “Scope 3,” which includes the supply chain and single-use medical devices is 80% of healthcare emissions. Supply chain challenges continue and reprocessing builds resilience into the supply for critical medical devices.
AMDR Suggested SUD Definition Language (US)
Although most SUDs cannot be safely re-used, some can be without added patient risk or loss of functionality. The reprocessing of SUDs requires strict FDA oversight and exhaustive documentation to prove to FDA that the SUD can be safely cleaned, tested, inspected, and reused.
In other words, the single-use label does not necessarily mean the SUD can only be used once.
AMDR Suggested SUD Definition Language (EU)
Although most SUDs cannot be safely re-used, some can be without added patient risk or loss of functionality. The remanufacturing of SUDs requires strict notified body and competent authority oversight and compliance with the EU Medical Device regulation to prove that the SUD can be safely cleaned, tested, inspected, and reused.
In other words, the single-use label does not necessarily mean the SUD can only be used once.